We use cookies, including cookies from third parties, to enhance your user experience and the effectiveness of our marketing activities. These cookies are performance, analytics and advertising cookies, please see our Privacy and Cookie policy for further information. If you agree to all of our cookies select “Accept all” or select “Cookie Settings” to see which cookies we use and choose which ones you would like to accept.
HVAC Systems Explained: Maintaining Comfort and Air Quality

HVAC systems are important for keeping our homes warm during cold seasons and making us feel cool during the hot season. HVAC stands for Heating, ventilation, and air conditioning systems. It's a collective term for all different cooling and heating systems used to change indoor temperature and humidity. It is designed to regulate the temperature, humidity, and air quality inside a building, ensuring a comfortable environment while minimizing energy consumption needs. Some common HVAC systems are air conditioning units, heat pumps, furnaces, etc.1)
Refrigerants are integral components in HVAC systems, ensuring smooth operation by causing cooling. As they circulate inside refrigeration devices, heat pumps, and air conditioners, refrigerants absorb heat from indoor air, carry it to the outdoor unit, and release it into the atmosphere. An essential component in this process is the compressor, which compresses the refrigerant, increasing its pressure and temperature, and enabling it to release heat outside. This function is critical for the operation of air conditioners and heat pumps, ensuring that the indoor environment remains comfortable.

Principles of Heat Transfer
Heat is an energy transfer resulting from a temperature difference between the system and its surroundings. There are two forms of heat flow: sensible heat and latent heat. Sensible heat refers to the heat used to change the temperature without changing the state of the material while latent heat is the amount of energy needed to cause a phase change. It might be from a gas to a liquid or from a liquid to a solid and back again and is often required to add or remove humidity in the air. Latent heat is an energy released or absorbed by a thermodynamic system during a constant-temperature process. Two common forms of latent heat are latent heat of fusion (melting) and latent heat of vaporization. It can be seen in the graph below when changing from one phase to the next: from solid to liquid, and liquid to gas.
The graph below shows the change in various forms of matter and the heat-related to each process.
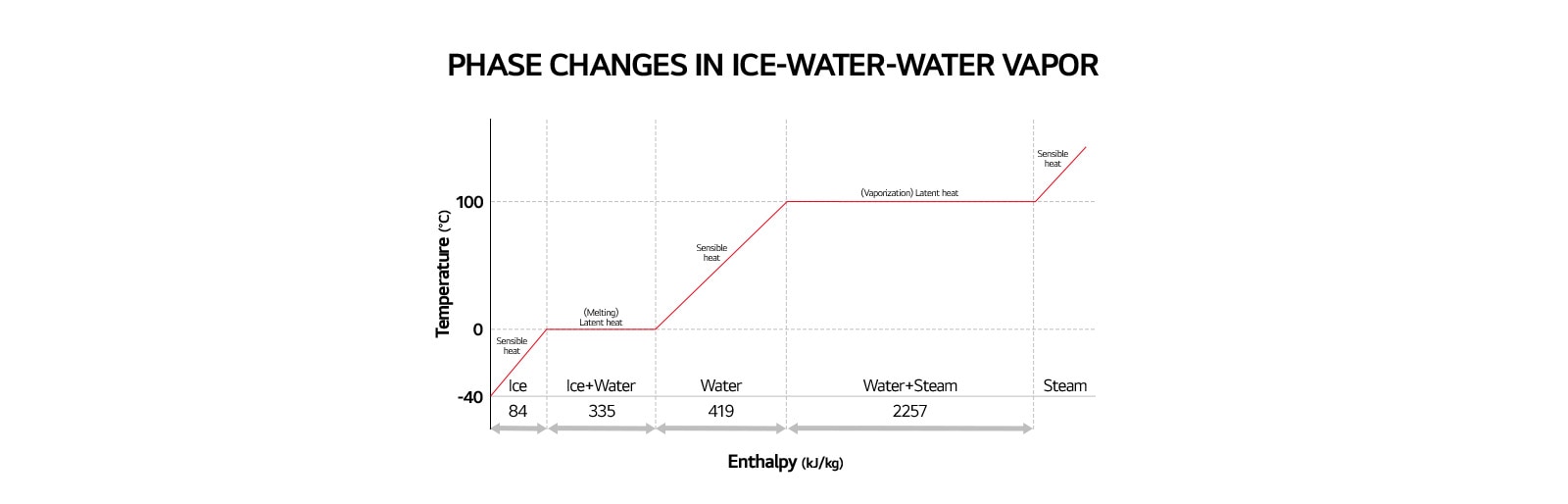
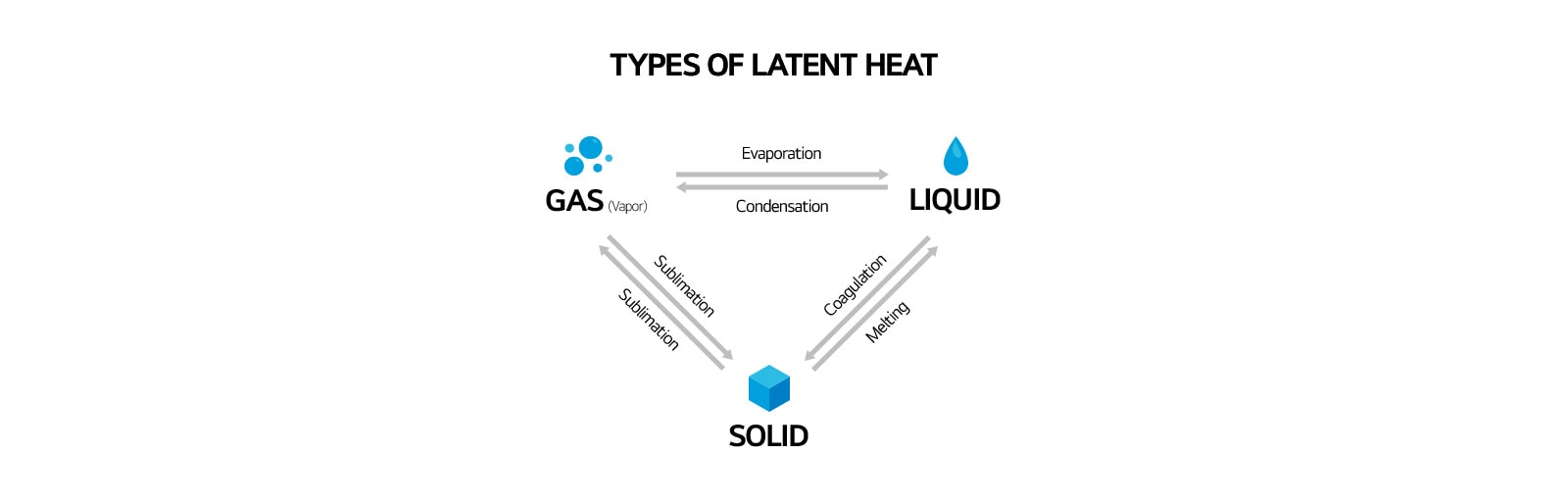
Properties of Air and Relative Humidity
Understanding the properties of air is also important. Air is a mixture of gases, 78% nitrogen and 21% oxygen with traces of water vapor, carbon dioxide, argon, and various other components. The amount of water vapor in the air that can be contained at a given temperature is limited. When air holds as much water vapor as it can for a given temperature, it is said to be saturated. It is also known as Relative humidity.
Relative humidity is the degree of humidity contained in the humid air. When the Relative humidity is 100%, the water vapor content in the atmosphere is saturated, and dew condensation occurs. The temperature at that time is called the ‘dew point temperature’.2) The higher the dew point rises, the greater the air's moisture.
Understanding of the Psychrometric Chart
To stay comfortable and cool indoors, maintaining suitable air humidity is important. It is generally recommended to keep the Relative humidity (RH) values indoors between 40 and 60% for comfort and health.3)
The psychrometric chart is a powerful tool for HVAC professionals and anyone interested in understanding indoor air comfort. It can be used to calculate the moisture content of air at various temperatures. It is also a graphical representation of the physical and thermal properties of atmospheric air for a particular barometric pressure. These thermal properties include Dry bulb temperature (DBT), Wet bulb temperature (WBT), Relative humidity, enthalpy, moisture content, and air density.4)
An example of the psychrometric chart
Dry bulb temperature is located on the horizontal, or x-axis of the chart. It is the actual air temperature measured by a thermometer and does not contain the moisture content in the air. Other important lines on the psychrometric chart include the Wet bulb temperature (WBT) lines, which run diagonally from the upper left to the lower right. The Wet bulb temperature is taken by surrounding the thermometer with a wet wick and the reading is recorded as the water evaporates. It accounts for the cooling effect of evaporation and is always lower than or equal to the Dry bulb temperature. Using a psychrometric diagram or Mollier chart, the state of the humid air can be determined by combining the Dry bulb and Wet bulb temperatures. Wet bulb temperature lines on the chart run diagonally from the upper left to the lower right.5)
The humidity ratio is the amount of water vapor in wet air and is expressed as the mass ratio of water vapor to 1 kg of dry air. The humidity ratio can be found on the vertical, y-axis with lines of constant humidity ratio running horizontally across the chart. Moist air also consists of heat energy. The measure of the total heat energy, absorbed or released is called enthalpy. It is also equivalent to the sum of total internal energy and resulting energy due to its pressure and volume.6)
Conclusion
In summary, HVAC systems are more than just mechanisms for temperature control; they are complex systems designed to balance multiple factors to improve indoor air quality and overall comfort. A thorough grasp of heat transfer principles, refrigerant functions, and air properties equips individuals with the knowledge needed to operate, and maintain HVAC systems ideally. As technology advances, continued learning and adaptation will be key to harnessing the full potential of HVAC systems and ensuring they meet evolving standards of comfort and efficiency.
1) Lutz, A. (n.d.). What Is HVAC and How Does it Work? (2024 Guide). Architectural Digest. https://www.architecturaldigest.com/reviews/hvac/what-is-hvac
2) The state of water vapour. (2024, April 2). RMetS. https://www.rmets.org/metmatters/state-water-vapour
3) Ness, M. C. A. (n.d.). Indoor relative humidity: relevance for health, comfort, and choice of ventilation system. Indoor Relative Humidity: Relevance for Health, Comfort, and Choice of Ventilation System. https://doi.org/10.4995/vibrarch2022.2022.15237
4) Yaciuk, G. (1981b). SOLAR CROP DRYING. In Elsevier eBooks (pp. 377–396). https://doi.org/10.1016/b978-0-08-025388-6.50049-0
5) Temperatures - Dry Bulb/Web Bulb/Dew Point. (n.d.). https://www.weather.gov/source/zhu/ZHU_Training_Page/definitions/dry_wet_bulb_definition/dry_wet_bulb.html
6) GeeksforGeeks. (2024, April 3). Enthalpy Definition, Formula and Reactions. GeeksforGeeks. https://www.geeksforgeeks.org/what-is-enthalpy/
7) GPJ Ajpur. (n.d.). Resource on Learning in Educational Studies. GPJ Ajpur. https://www.gpjajpur.org/public/uploads/lres-770.pdf
8) Chembook. (n.d.). Chapter 5: Chemical Properties and Reactions. http://chembook.org/page.php?chnum=5& sect=8
9) Broughton EAP. (2019). PSIG and BTU Comparison Image. Broughton EAP. https://www.broughtoneap.co.uk/wp-content/uploads/2019/09/psig-and-btu.jpg
- Previous
- Next
The URL has been copied to the clipboard.